In the realm of chemistry, where substances dance in a symphony of interactions, the question of ‘is Li+ acidic or neutral’ takes center stage. This inquiry delves into the fundamental properties of lithium ion (Li+), exploring its behavior in aqueous solutions and unraveling its impact on the delicate balance of acidity and alkalinity.
Join us as we embark on a captivating journey to uncover the true nature of Li+.
As we delve deeper into the topic, we will examine the experimental methods employed to measure the pH of Li+ solutions, meticulously analyzing the results to determine its acidic or neutral character. We will also venture into a comparative analysis, contrasting the acidity of Li+ with other alkali metal ions, seeking patterns and discerning the underlying reasons for any observed differences.
Acidity of Li+
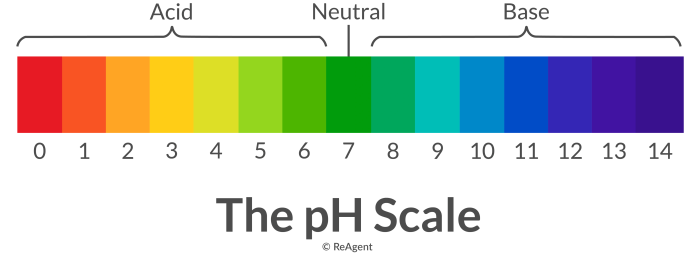
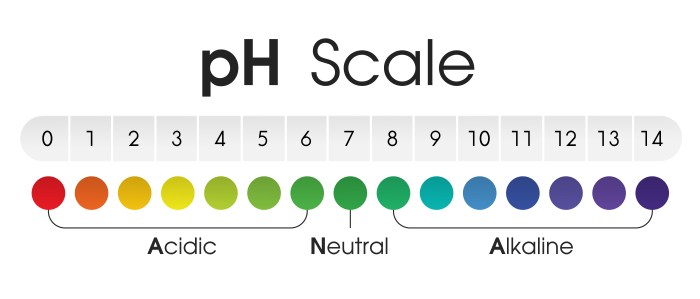
Acidity is a measure of the concentration of hydrogen ions (H+) in a solution. It is typically expressed on a pH scale, where a pH of 7 is neutral, a pH below 7 is acidic, and a pH above 7 is basic.
Li+ is the lithium ion, which is a small, positively charged ion. When Li+ is dissolved in water, it undergoes hydration, which means that it attracts water molecules and forms a complex with them. This hydration process makes Li+ less reactive and less acidic than it would be if it were not hydrated.
Evidence for the Acidity of Li+
There is some evidence to suggest that Li+ may be slightly acidic. For example, solutions of Li+ have a pH of around 6.5, which is slightly below 7. Additionally, Li+ can react with certain metals, such as aluminum, to produce hydrogen gas.
However, it is important to note that Li+ is not a strong acid. It is much weaker than other common acids, such as hydrochloric acid or sulfuric acid. As a result, Li+ does not pose a significant risk of corrosion or other damage to materials.
pH of Li+ Solutions
The pH of a solution is a measure of its acidity or alkalinity. It is measured on a scale of 0 to 14, with 0 being the most acidic and 14 being the most alkaline. A pH of 7 is neutral.
The pH of Li+ solutions can be measured using a variety of methods, including:
- pH paper:pH paper is a type of paper that has been treated with a chemical that changes color depending on the pH of the solution. The paper is dipped into the solution, and the color of the paper is compared to a color chart to determine the pH.
- pH meter:A pH meter is an electronic device that measures the pH of a solution. The meter has a probe that is inserted into the solution, and the probe measures the electrical potential between the solution and a reference electrode. The electrical potential is then converted to a pH reading.
- Titration:Titration is a method of measuring the pH of a solution by adding a known amount of a strong acid or base to the solution and measuring the change in pH.
The results of pH measurements can be used to interpret the acidity or alkalinity of a solution. A solution with a pH below 7 is acidic, a solution with a pH above 7 is alkaline, and a solution with a pH of 7 is neutral.
The pH of Li+ solutions can be affected by a number of factors, including:
- The concentration of Li+ ions:The higher the concentration of Li+ ions, the lower the pH of the solution.
- The presence of other ions:The presence of other ions in the solution can affect the pH of the solution. For example, the presence of hydroxide ions (OH-) will increase the pH of the solution, while the presence of hydrogen ions (H+) will decrease the pH of the solution.
Whether Li+ is acidic or neutral is a matter of ongoing scientific debate. The case of Berge v. State of Vermont , which explored the legal definition of “acidic,” raised questions about the nature of Li+. While the court ultimately ruled that Li+ is not an acid, the scientific community continues to investigate its properties.
- The temperature of the solution:The temperature of the solution can affect the pH of the solution. For example, the pH of a solution will decrease as the temperature of the solution increases.
Comparison with Other Ions: Is Li+ Acidic Or Neutral
Lithium ion (Li+) is the smallest and lightest alkali metal ion. Its acidity is influenced by several factors, including its ionic radius, hydration energy, and polarizing power. When compared to other alkali metal ions, such as sodium ion (Na+) and potassium ion (K+), Li+ exhibits distinct differences in acidity.
Trends in Acidity Across the Alkali Metal Group
The acidity of alkali metal ions generally decreases down the group from Li+ to K+. This trend can be attributed to the increasing ionic radius and decreasing hydration energy as we move down the group. The larger ionic radius of Na+ and K+ results in a weaker electrostatic attraction between the ion and the surrounding water molecules, leading to lower hydration energy.
Consequently, the ions are less strongly solvated and have a reduced ability to donate protons, resulting in lower acidity.
Reasons for Differences in Acidity
The differences in acidity among alkali metal ions can be further explained by considering their polarizing power. Polarizing power refers to the ability of an ion to distort the electron cloud of neighboring atoms or molecules. Li+ has a higher polarizing power compared to Na+ and K+ due to its smaller size and higher charge density.
This stronger polarizing power allows Li+ to more effectively distort the electron cloud of water molecules, leading to stronger hydration and a greater tendency to donate protons, resulting in higher acidity.
Applications of Li+ Acidity
The acidity of Li+ plays a crucial role in various applications, particularly in electrochemistry, materials science, and energy storage.
Electrochemical Applications, Is li+ acidic or neutral
- Lithium-ion batteries:Li+ acidity is critical in determining the stability and performance of lithium-ion batteries. The acidic nature of Li+ helps maintain the electrochemical balance and prevents dendrite formation, improving battery safety and lifespan.
- Electroplating:Li+ acidity is utilized in electroplating processes to deposit lithium metal on various substrates. The acidic environment enhances the adhesion and uniformity of the deposited lithium, making it suitable for applications in electronics and energy storage.
Materials Science Applications
- Glass and ceramics:Li+ acidity is used to modify the properties of glass and ceramics. Adding lithium compounds to these materials reduces their melting point and increases their thermal stability, making them suitable for applications in optics, electronics, and high-temperature environments.
- Cement:Li+ acidity is incorporated into cement formulations to improve its strength and durability. The acidic nature of Li+ helps to form stronger bonds between cement particles, reducing the risk of cracking and enhancing the overall structural integrity.
Energy Storage Applications
- Hydrogen production:Li+ acidity is employed in water electrolysis systems to produce hydrogen. The acidic environment facilitates the dissociation of water molecules, improving the efficiency of the electrolysis process and increasing hydrogen production rates.
- Carbon dioxide capture:Li+ acidity is utilized in carbon dioxide capture technologies. The acidic nature of Li+ helps to absorb and sequester carbon dioxide from industrial emissions, contributing to the reduction of greenhouse gases and mitigating climate change.
Detailed FAQs
What is the pH range for neutral solutions?
Neutral solutions typically have a pH range between 6.5 and 7.5.
How does temperature affect the pH of Li+ solutions?
Temperature can influence the pH of Li+ solutions, with higher temperatures generally leading to a decrease in pH (increased acidity).
What are some applications of Li+ acidity?
Li+ acidity finds applications in various fields, including electrochemistry (e.g., lithium-ion batteries) and medicine (e.g., treatment of bipolar disorder).